How to Calculate Relative Molecular Mass
For example let us calculate the relative molecular. It is often shortened to RMM.

Question Video Calculating The Relative Molecular Mass Of Xenon Difluoride From Its Chemical Formula Nagwa
1 Define the terms relative molecular mass and relative formula mass 2 Calculate the Mr of various molecules.

. 1007 x 2 2014 grams per mole. 379K subscribers At the end of this video you should be able to. M r Average mass of a molecule x 12 Mass of an atom of 12 C where M r - relative molecular mass Beer-Lambert Law Science Calculators Blue-Shift Velocity.
This is calculated from the mass number and relative abundances of all the isotopes of a particular element. It could also be calculated this way. Relative mass of an element is calculated by adding the number of protons to the number of neutrons for the specific isotope of the element.
01348 g of gas was found to occupy a volume of 2580 ml at 0 OC and 760 mm of Hg pressure. There are two ways to calculate the RMM here. The relative molecular mass is 6600.
Enter any chemical formula optionally with parenthesis and crystal water as in this example. Write a mathematical expression to calculate the total mass of all the elements in the compound called the relative molecular mass of the compound Mr X a Y b relative. The symbol for the relative formula mass is Mr and it refers to the total.
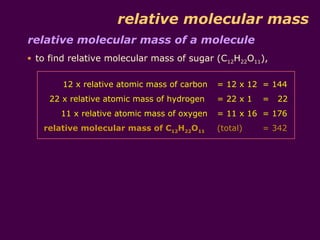
Calculation of relative mass of. Relative molecular mass of a molecule Average mass of one molecule of a substance mass of d f r a c 1 12 of an atom of carbon 12. It is calculated by adding up the relative atomic masses A r of all the atoms present in.
To calculate the relative atomic mass Ar of chlorine. Some elements are only found in molecules of 2 or more atoms. Relative Molar Mass Calculator for Chemists.
Scientists call this the relative molecular mass. A_ r frac totalmassofatoms totalnumberofatoms frac 75 times 35 25 times 37 7525 A_ r frac. Weighted average of the atomic masses of its isotopes and their relative abundances Relative because compared to 1 atom of carbon-12 12 C which is 12 units.
In this video we look at how to work out the mass of molecules. They have no units because they are relative masses. Multiply the relative atomic mass by the molar mass constant.
Ether has the formula CH 3 CH 2 OCH 2 CH 3. The Relative Molecular Mass of a compound is the sum of the masses of all the atoms present in the molecule. I show you how to work this out and then ex.
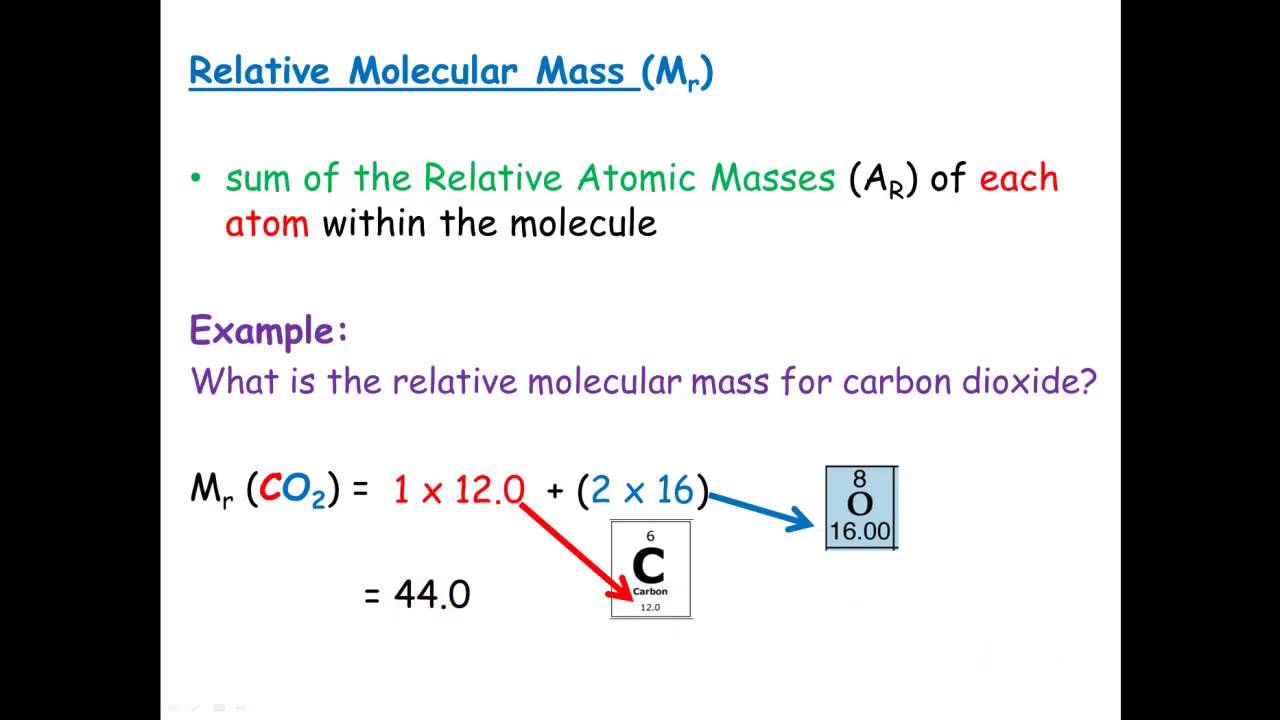
Mr of CO2 1 12 2 16 12 32 44 Like Ar values Mr values are just numbers. What is the formula of relative molecular mass. Relative atomic mass Ar.
The RMM is used in many sorts. Relative formula mass M r is mass of a molecule or compound on a scale compared to carbon-12. The relative molar mass symbol M r is a dimensionless quantity related to the molar mass M by M r M10 3 kgmol 1.
OR The method on the left takes each small group in the molecule and works out its mass first.
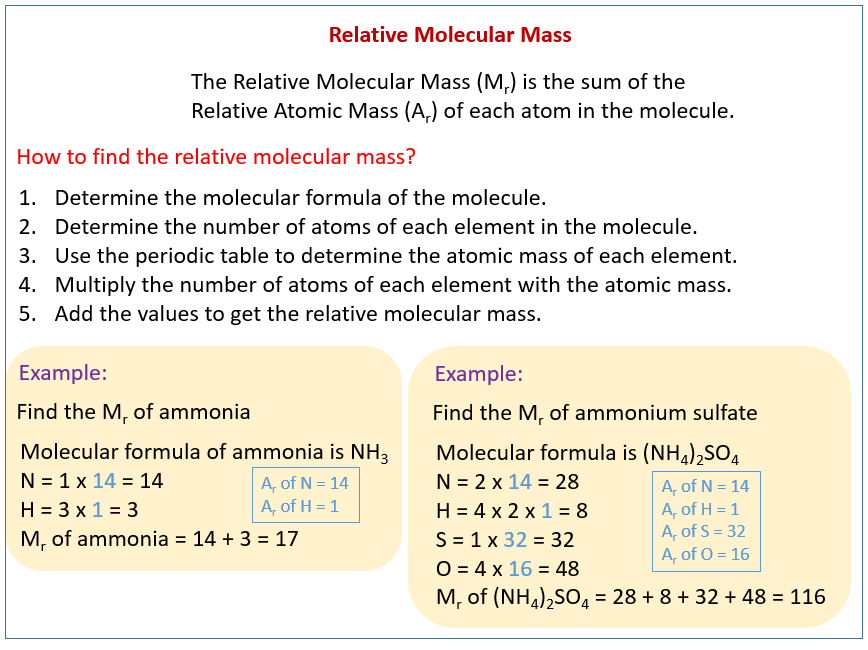
Relative Molecular Mass Relative Formula Mass Video Lessons Examples And Solutions

Relative Molecular Mass Chemical Formula And Equation Youtube
0 Response to "How to Calculate Relative Molecular Mass"
Post a Comment