Which Solution Has the Highest Total Concentration of Ions
From the given options an acid will have highest concentration of H 3 O ions. The number in the pH is equal to the negative exponent of 10.

Calculating Ion Concentration In Solutions Chemistry Tutor Youtube
You know there are 2 moles of H ions the active chemical species in an acid-base reaction for every 1 mole of sulfuric acid.
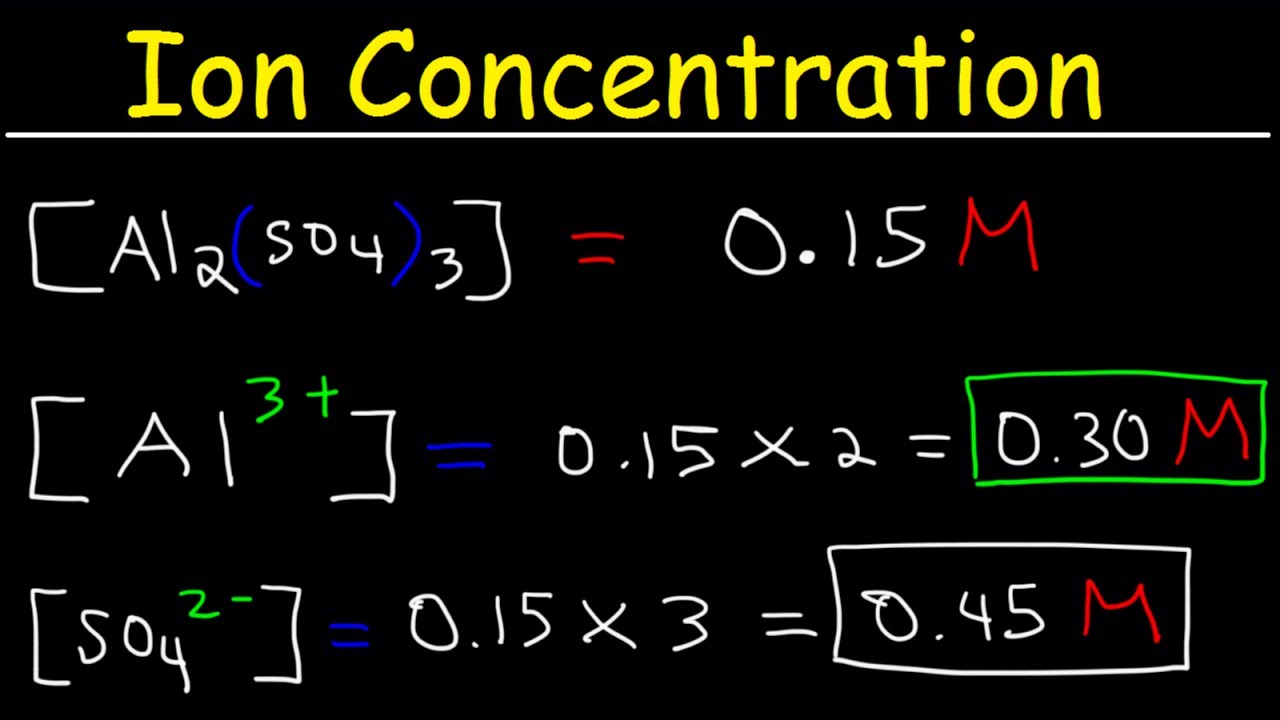
. PH 35 B. To know which of the given solutions has the highest concentration of chloride ions we must evaluate each of them. Which of the following solutions contains the highest total concentration of ions assuming complete dissociation.
0 2 M H N O 3 will give highest concentration of H 3 O ions since it is an acid. PH 78 C. Mar 19 2013.
I know the most acidic solution has the lowest pH and the most basic solution has the highest pH. In general M 1 usually refers to as the initial molarity of the solution. The equivalence point is reached when all of the Haq ions in the HCl solution have reacted.
V 1 refers to the volume that is being transferred. The color change signals the endpoint of the titration. 1 010 M MgCl₂.
040 M MgCl2 040 M CaCl2 020 M NaCl 060 M AlCl3 All of these solutions have the same concentration of chloride ions. This video contains plenty of examples and practic. We can see that in 1 mol of MgCl₂ we have 1 mol of.
PH 124 D. Solution for Which solution below has the highest concentration of hydroxide ions. The volume units must be the same for both volumes in this equation.
So the lowest pH will have the highest H ion concentration. Which one of the following aqueous solutions would have the higher concentration of K aq ions. Thus its concentration would be 5 times larger.
13 Which has the greater concentration of hydrogen ions a solution with a pH of 5 or a solution with a pH of 4. Get the full course at. 0 2 M H N O 3 will give highest concentration of H 3 O ions since it is an acid.
The largest concentration would be 0010 M aluminum sulfate because it produces 5 ions when dissociated. Which of the following solutions will have the highest concentration of chloride ions. Sulfuric acid is a strong acid that completely dissociates into its ions H and SO 4 2- in aqueous solution.
It dissociates in water and gives 3 ions one ion of barium and two ions of hydroxide with the concentration of 0008 M for each one. PH 982 pH 126 pH 793 pH 321 pH 700. RbOHaq NH3aq HFaq HBraq CaOH2aq.
So the solution of barium hydroxide is a stronger electrolyte as it has a higher concentration of dissociated ions. Indicators change color when the hydrogen ion concentration of a solution changes substantially. Chemistry questions and answers.
10-3 is bigger then 10-9. A 0010 M AI2 SO43 b 0010 M AICl3 c 0020 M NaCl d 0010 M CaCl2. So if you have a compound that dissociates into cations and anions the minimum concentration of each of those two products will be equal to the concentration of the original compound.
5001 0050 M. Remeber that the number of moles of solute does not change when more solvent is added to. Which solution below has the highest concentration of hydronium ions H.
025 M Ba3PO42 025 M Na2504 025 M ANO33 025 MKCI This problem has been solved. Molarity is measured in number of moles of a. M 2 refers to the final concentration of the solution and V 2 is the final total volume of the solution.
This worked example problem illustrates the steps necessary to calculate the concentration of ions in an aqueous solution in terms of molarityMolarity is one of the most common units of concentration. See the answer See the answer See the answer done loading. 2 ions of Al³ and 3 ions of SO₄².
The concentration of ions in solution depends on the mole ratio between the dissolved substance and the cations and anions it forms in solution. Which of the following solutions has the highest total concentration of ions. PH 51 E.
From the given options an acid will have highest concentration of H 3 O ions. The other options contain base K O H M g O H 2 and an organic species C 6 H 1 2 O 6 which will have low H 3 O ion concentration. This chemistry video tutorial explains how to calculate the ion concentration in solutions from molarity.
The consumption of Haq can be detected by employing a chemical dye known as an indicator. This makes the total concentration of ions to be 0024 M. From the given options the solution of 010M AlCl₃ is the one that has the highest concentration of chloride ions.
Assuming equal concentrations rank these solutions by pH. So the answer is ph321. Heres how that works.
Assume total solubility in water A 10 M KC2H3O2 B 10 M KNO3 C 10 M K2CO3 D 10 M K3PO4 E all of the above. 15 Where is the highest concentration of hydrogen ions in the chloroplast. Which of the following solutions contains the highest total concentration of ions assuming complete dissociation.
While the total concentration of K I is 002 M. Assume that the pH of a soil solution is 79 and the total concentration of Ca Ca Tis 3715 10 3 mol liter 1. Which solution below has the highest concentration of hydronium ions.
43 and conditional equilibrium constants can be used to speciate a metal ion in a soil solution let us calculate the concentration of the different Ca species present in a typical soil solution from a basic soil. 14 Who gave the measure of hydrogen ion concentration. The other options contain base K O H M g O H 2 and an organic species C 6 H 1 2 O 6 which will have low H 3 O ion concentration.
So a pH of 7 has a concentration where the exponent is -7 a pH of 9 will have an exponent equal to -9 and so on.
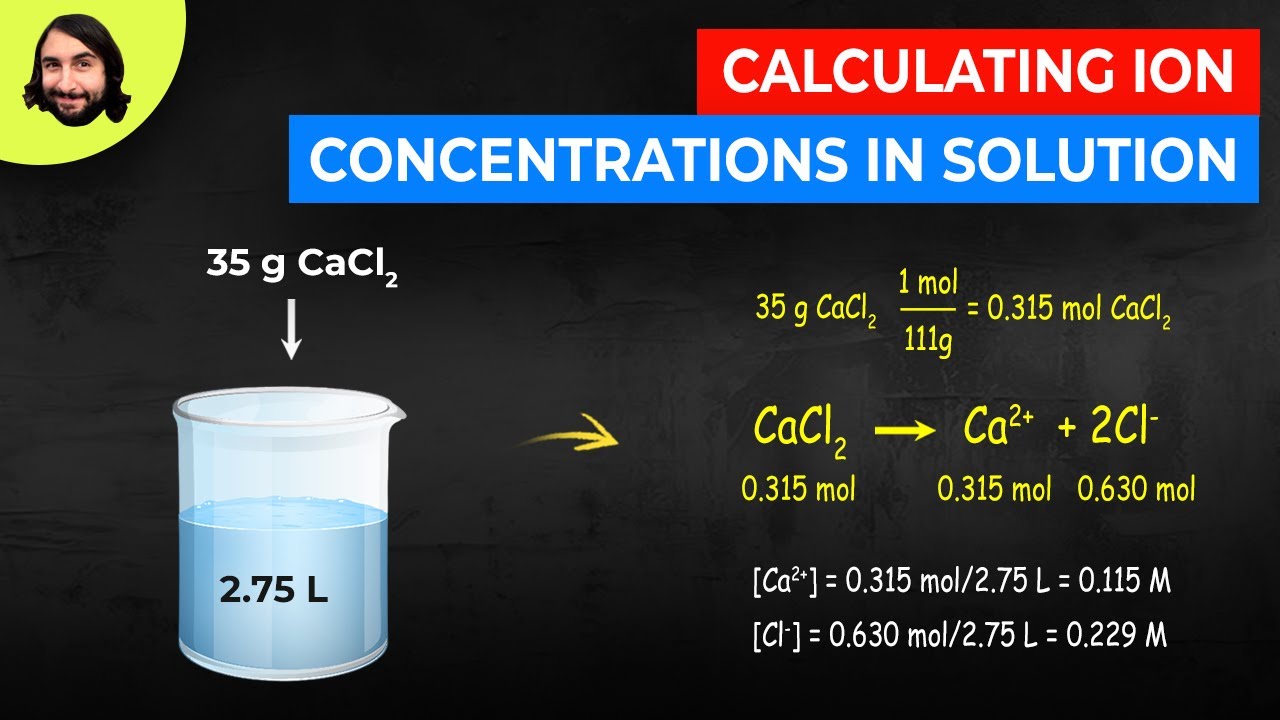
Calculating Ion Concentrations In Solution Youtube

Pin By Homework Az On Chemistry Solutions Answers Concentration

Ion Concentration In Solutions From Molarity Chemistry Practice Problems Youtube
0 Response to "Which Solution Has the Highest Total Concentration of Ions"
Post a Comment